Using genetics to conserve life history strategies in California steelhead (Oncorhynchus mykiss)2/4/2021
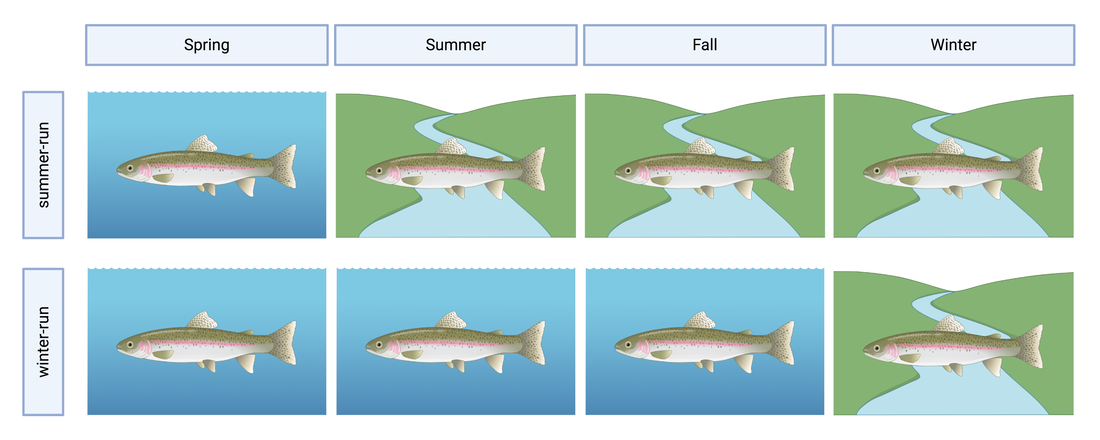
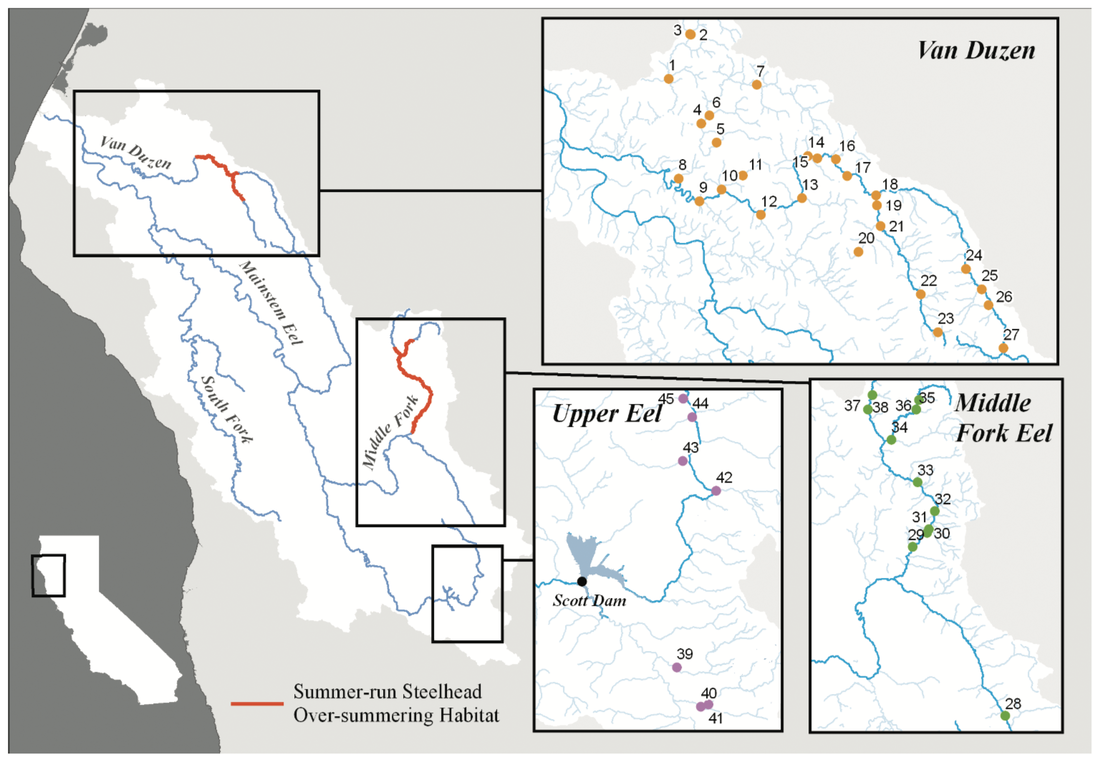
This blog post was originally published by the AGA Blog in February 2021. By Alexandra L. DeCandia Steelhead (Oncorhynchus mykiss) inhabit cultural and ecological niches in northern California and the Pacific Northwest. Culturally, steelhead provide an important food source to indigenous communities and represent abundance, wealth, prosperity, and determination in traditional art and religious ceremonies (Squamish Lil’Wat Cultural Center). Ecologically, migratory steelhead transport key nutrients between marine and freshwater environments (and the organisms that inhabit them) and serve as indicator species for ecosystem health. Diverse life history strategies adopted by steelhead are critical to the fulfillment of these niches. Perhaps most emblematic concerns migration. Steelheads are classified into two primary groups: residents and migrants. Resident individuals inhabit streams, rivers, lakes, and reservoirs throughout their lives, whereas anadromous steelhead migrate between marine and freshwaters for feeding and spawning. Within the migrating group, there are additional strategies regarding the timing of migration from marine to freshwaters (termed “run-timing”; Figure 1). Summer-run steelhead migrate to freshwaters in late spring/early summer to mature in the fall and spawn in winter. In contrast, winter-run steelhead wait until late fall/winter to migrate into freshwaters for spawning. Although once abundant throughout the region, steelhead have experienced dramatic declines due to anthropogenic activities. Stressors include dams, diversions, development, pollution, run-off, harvest, thermal stress, and reduced stream flow that render certain routes impassable. In addition to causing the loss of individuals, these threats jeopardize the complex life histories exhibited by steelhead in the region. Summer-run steelhead are particularly vulnerable and at risk of local extirpation. In their recent study, Samantha Kannry et al. (2020) examined the distribution of life history strategies in the southernmost river system with summer-run steelhead: the Eel River in northern California (Figure 2). More specifically, the authors used genetic techniques to examine population structure, demography, and ecology throughout the Eel River watershed. Their dataset consisted of genome-wide loci genotyped through Rapture sequencing, as well as markers previously associated with migration (OMY5) and run-timing (GREB1L). Their sample set included more than two thousand steelheads sampled for this and a previous study in the Eel River watershed. Principal component analysis revealed three genetic clusters within the watershed. Interestingly, steelhead primarily clustered by sampling basin rather than life history phenotype. Summer-run and winter-run individuals sharing the same sampling basin (e.g., Van Duzen) therefore clustered more closely together than summer-run steelheads sampled across basins. This result supported the presence of two independent populations of summer-run steelhead in the Van Duzen and Middle Fork Eel Rivers. Critically, this overturned the previous assumption that all summer-run steelhead derived from the same subpopulation. Kannry et al. next considered potential barriers to steelhead passage found throughout the watershed. Through examination of migration allele frequencies, they reported that Eaton Falls does not present a complete barrier to anadromous steelhead, as previously thought. This result was supported by estimates of population differentiation across no barrier (mean pairwise FST=0.059), the partial Eaton Falls barrier (0.094), and the confirmed South Fork barrier (0.219). Within migrating steelhead, they also identified barriers between winter-run and summer-run steelhead in both the Van Duzen (Salmon Falls barrier) and Middle Fork Eel (Osbourne Roughs barrier) rivers, suggesting habitat segregation between the two run-timing strategies. This result found its extreme in the South Fork Eel River, where no summer-run alleles were identified, thus indicating the absence of this strategy from this part of the watershed. The final barrier considered by the authors was Scotts Dam, a hydropower facility that has diverted water into the nearby Russian River watershed for roughly 100 years. Constructed without a fish passage, Scotts Dam presents a formidable barrier to anadromous steelhead. As this dam is up for relicensing in 2022, Kannry et al. considered whether the migration phenotype could be recovered above the dam. Through population genetic surveys, the authors identified both anadromous and summer-run alleles in the resident steelhead population sampled above Scotts Dam. This suggests that steelheads may be able to naturally recover this important strategy without human intervention, should the dam be removed. In summary, the authors identified distinct population segments, mixed and distinct resident and migrant waters, candidate areas for habitat restoration, and potential source populations for the natural recovery of life history strategies. These insights can directly inform the conservation and management of steelhead in the Eel River watershed, particularly regarding the 2022 Scotts Dam relicensing. In addition to these implications, the analytical approach described by Kannry et al. provides a roadmap for conducting similar analyses in other river systems in northern California and the Pacific Northwest. By better understanding the ecology and distribution of life history strategies throughout the region, the authors demonstrate that diversity can be preserved. Given steelhead’s significance, these analyses and management recommendations may enable steelhead to occupy important cultural and ecological niches indefinitely. This blog post was originally published by the AGA Blog in November 2020. By Alexandra L. DeCandia Sea turtles and their ancestors have roamed the world’s oceans for more than 100 million years. Over the course of millennia, these living dinosaurs developed an intricate life cycle containing mysteries we have yet to solve. This cycle begins at beach rookeries. Guided by starlight, hatchlings race towards the sea as soon as they emerge from their sand-covered nests. They swim continuously for days and enter a period of life called “the lost years”, where they develop far from coastlines and predators. Years later and many pounds heavier, juvenile turtles migrate to near-shore foraging grounds until they reach sexual maturity, when they return to their natal beaches. After mating (often with multiple partners), females haul their heavy bodies to shore, bury their clutches, and return to sea – restarting this decades-long cycle (SWOT). The migratory lifestyle of sea turtles, although stable for millennia, now exposes these ocean nomads to innumerable anthropogenic threats. These include climate change, fisheries bycatch, loss of coastal habitat, pollution, and exploitation for the illegal wildlife trade (WWF). As numbers fall, the additional threat of interspecific hybridization emerges within shared mating and nesting grounds. Sea turtle species diverged from one another between 20-100 million years ago; thus hybridization may lead to outbreeding depression, where hybrids and their offspring suffer from reduced fitness or reproductive success. As a result, it is critically important to identify hybridization hot spots and study the effects of these interspecific unions. The northeastern coast of Brazil is one such hot spot. Coastlines within the state of Bahia contain large-scale rookeries of vulnerable loggerhead (Caretta caretta) and critically endangered hawksbill (Eretmochelys imbricata) turtles. Similar overlap is observed in the state of Sergipe between loggerhead and vulnerable olive ridley (Lepidochelys olivacea) turtles. In some locations, the frequency of hybrids has reached as high as 42% within the last few decades, rendering hybridization a serious cause for concern in this region. The first step towards addressing this concern is hybrid detection. Traditional methods rely on a handful of mitochondrial or nuclear markers to identify F1 (e.g., loggerhead x hawksbill) and F2 (e.g., F1 hybrid x hawksbill) etc. hybrids. However, these methods possess high error rates and lack fine-scale resolution. To overcome these limitations, Arantes et al. (2020) developed a highly informative multilocus panel of genomic markers to identify sea turtle hybrids. Drawn from reduced representation ddRAD data, the panel consists of loci with high intra- and interspecific variation to enable surveys of population structure as well as hybrid detection. To minimize ascertainment bias, the authors used Sanger sequencing to analyze variation as phased haplotypes. The dataset contained samples from five species of sea turtles, including loggerhead, hawksbill, olive ridley, green (Chelonia mydas), and leatherback (Dermochelys coriacea) turtles. The multilocus panel effectively differentiated between the five species included in this study and was even able to detect fine-scale population structure within species. It additionally identified 29 F1 hybrids through their intermediate genomic composition. These included 15 loggerhead x hawksbill hybrids, 12 loggerhead x olive ridley hybrids, and two hawksbill x olive ridley hybrids. Interestingly, only six >F1 hybrids were identified, with all six samples collected from hatchlings. Four were even sampled from the same nest, with two containing loggerhead and hawksbill ancestry, and two containing ancestry from loggerhead, hawksbill, and green sea turtles! Considered together, these results (and this clutch in particular) exemplify the complicated nature of sea turtle hybridization. They further highlight the utility of high-resolution genomic techniques for informing wildlife conservation. The panel developed by Arantes et al. (2020) provides a valuable tool for the study and management of sea turtles – not only in Brazil, but around the world. Future work can identify other hybridization hotspots and assess the risk of outbreeding depression in F1 and >F1 hybrids. Analyses of hybrid diet, health, fitness, behavior, and genomics can all help ascertain that risk. This will become increasingly important as sea turtle populations continue to decline worldwide, leading to higher rates of hybridization. Ultimately, the best way to reduce hybridization is to halt these declines. But to do so, we’ll need to mitigate climate change, promote sustainable fisheries, save coastal habitat, minimize pollution, and end the illegal wildlife trade. The task is daunting, but the outcome is worthwhile: sea turtles roaming the oceans for millennia to come. This blog post was originally published by the Friends of the Island Fox in April 2020. By Alexandra L. DeCandia Over the last few decades, we've realized that organisms are far more complicated than they initially appear. What may look like an individual fox is actually an ecosystem containing trillions of microorganisms on every square inch. Despite their tiny size, microbes influence important host functions, such as development, digestion, stress tolerance, behavior, and even immunity. Therefore learning more about these hidden actors can inform wildlife conservation of at-risk species in the modern molecular era. Microbes may be particularly important to species that lack genetic diversity, such as Channel Island foxes, especially where disease threatens long-term persistence. On Santa Catalina Island, scientists discovered extremely high rates of ear canal tumors, where roughly half of adult foxes have growths in their ears. Although the exact cause is unknown, researchers linked ear mite infection to tumor growth and development. The most prominent hypothesis states that infection with ear mites leads to inflammation and rampant cell growth in the ear canal, which in turn leads to tumors. Thankfully, treating foxes with the acaricide Ivermectin has already decreased mite burdens and tumor rates in these foxes. However, there's more to this story. We still don't fully understand how mite infection leads to tumor growth. In particular, my collaborators and I wondered whether microbes play a role in this system. For example, do mites disrupt healthy microbes and cause secondary bacterial infections? And do those infections then contribute to the chronic inflammation that precedes tumor growth? To address these questions, my collaborators at the Catalina Island Conservancy collected microbe samples by swabbing ear canals (and a few other body sites) of healthy and mite-infected foxes. (This process is similar to cleaning your ears with a cotton swab, except you don't throw away the swab afterwards.) Once a bunch of foxes were swabbed, all samples were sent to New Jersey, where I extracted DNA, collected genetic sequences, and analyzed the data. The results came back loud and clear: microbes differed between mite-infected and uninfected ear canals. Rather than a rich community of diverse microbes (as seen in healthy ears), mite-infected ear canals had fewer microbial species present. We further found that the balance of microbes (know as "relative abundance") differed between infection groups. As it turned out, this pattern was almost entirely driven by an overabundance of one bacterial species: Staphylococcus pseudintermedius (Class: Bacilli). Even though this microbe is commonly found on canid species (such as domestic dogs and foxes), it can become an opportunistic pathogen when healthy communities are disrupted. Once it proliferates, it can be incredibly difficult for the immune system or even antibiotics to eradicate, leading to chronic inflammation. We now hypothesize that mite infection and secondary bacterial infection with Staphylococcus pseudintermedius contribute to chronic inflammation and tumor growth in Santa Catalina Island foxes. Although further tests are needed to definitively establish causation, these insights into the microbial dynamics of mite infection can help us monitor the population for antibiotic resistant forms of Staphylococcus pseudintermedius that could cause a disease outbreak. They can further help us explore other open questions, such as why Santa Catalina Island foxes are the only subspecies with ear canal tumors, despite ear mites on other islands. As always in science, answers lead to more questions. But at least one thing is clear: there's more to this story (and indeed, to all organisms) than what initially meets the eye. This blog post was originally published by the Ecology and Evolution Blog in February 2019. By Alexandra L. DeCandia True to its name, the Anthropocene is characterized by human-mediated environmental change on a global scale. Currently, over 7.7 billion humans reside on our planet, 55% of whom live in cities. As a result, an estimated 3% of earth’s land surface is considered urbanized, with all of these values expected to increase in the next century [1]. As natural lands are coopted for human habitation and resource production, wildlife are left with three options [2]:
In each case, urbanization can raise conservation concerns or increase the potential for human-wildlife conflict. Thus, understanding the ecology and evolution of urban wildlife systems becomes critical for peaceful and long-term coexistence between humans and our neighboring wildlife. Red foxes (Vulpes vulpes) are an ideal study system for examining the effects of urban wildlife colonization. These flexible, mid-sized carnivores are capable of thriving in diverse habitats from natural landscapes through dense city centers [3]. Found across the northern hemisphere, urban fox colonization is especially pronounced in Europe, where foxes have successfully colonized cities since the 1930s. Aspects of their ecology, such as dietary preferences, disease transmission, and movement patterns, have been studied for decades. Yet there are still open questions about the consequences of urban fox colonization, both in terms of ecology and evolution. To better understand the evolutionary side of things, my collaborators and I set out to characterize the genetic effects of a relatively recent urban colonization event [4]. In the mid-1980s, red fox sightings in Switzerland increased dramatically after rabies was successfully eradicated. As rural populations expanded, more and more foxes moved into the Zurich metropolitan area [5]. Interestingly, movement studies showed that foxes settled in rural and urban areas tended to stay within one habitat type – even if they lived right on the border. That meant that rural foxes typically stayed in rural areas, and urban foxes typically stayed in urban ones. Since genetics are the raw material of evolution, understanding patterns of genetic diversity in these rural and urban foxes can provide insights into fox demography, colonization history, and chances of long-term survival. Thus in 2003, researchers used eleven neutral markers – or non-coding DNA with no known function – to describe the genetics of this so-called “city fox phenomenon” [6]. They reported decreased genetic diversity in urban foxes, and hypothesized that Zurich was colonized through two independent founder events: one east and one west of Lake Zurich, the Limmat River, and the city center. Though this study was among the first to examine the genetics of urban colonization, it was unable to consider potential adaptive differences between rural and urban foxes due to its use of neutral genetic markers. This may gloss over functional changes that occur to better suit unique aspects of each environment. For example, city foxes may experience different pressures than rural foxes, such as greater human presence, light and noise pollution, anthropogenic food availability, and disease exposure. Especially if foxes remain localized to one habitat type, these pressures may lead to selection on different behavioral, metabolic, or immune pathways in urban versus rural foxes. In our study, we revisited the Zurich city fox phenomenon with expanded datasets to consider the neutral and potentially adaptive consequences of urban colonization. We used nine genetic markers linked to functional immune genes and another 10,149 markers found throughout the genome. Our samples included resident foxes living in two urban and three rural locations in the Zurich metropolitan area. Consistent with previous movement and neutral genetic data, we found evidence of population structure between rural and urban foxes. In essence, foxes sampled in each area were more similar to one another than they were to foxes sampled elsewhere. We also observed that foxes east of the center-city landmarks (i.e., Lake Zurich, the Limmat River, and urban infrastructure) were distinct from foxes sampled to the west. This suggested that foxes don’t often cross those barriers to mate with individuals on the other side. In addition to population structure, we found decreased diversity in urban foxes when compared to rural foxes. Patterns of diversity at the genome-wide loci suggested a recent genetic bottleneck, whereby a large population quickly reduces in size (as is typical of founder events) and consequently loses variation. (Think of marbles in a bucket: if you have 150 marbles of different colors but only pull out 20 for a small jar, you can’t possibly capture all of the variation of the larger bucket. Genetic bottlenecks work similarly.) Despite these diversity losses, we observed evidence for balancing selection (which maintains variation) at markers linked to immune genes. This may be due to the selective advantage that comes from diversity, which can enable better recognition and response to different diseases [7]. We also found a number of genome-wide markers that were associated with urbanization. Interestingly, these markers were found in genes with functions related to metabolism, drug tolerance, immune processes, and colonization relevant behaviors (such as exploration, movement, circadian rhythms, and fear). These results may suggest local adaptation during urban colonization. As such, they provide a launching point for future studies looking for genes or gene functions that commonly recur across urban colonization events. By examining larger datasets (e.g., full genome sequences) in diverse species (from insects to reptiles to mammals etc.) around the world, we can discover the common ecological and evolutionary processes that characterize urban colonization. We can also identify unique challenges that urban environments pose to wildlife along the urbanophobe to urban exploiter spectrum. This will enable better monitoring and management of urban wildlife populations in the Anthropocene, and facilitate peaceful coexistence with our local wildlife in perpetuity. Works Cited
This blog post was originally published by Columbia Science Review in April 2015. By Alexandra L. DeCandia Monarch butterflies are an iconic American species. Found in all 50 states, these orange-and-black backyard visitors delight children with their delicacy and grace. They pass through our gardens each year, participants in an annual, multi-generational migration among the farthest undertaken by an insect species. Traveling south from Canada and the United States, eastern monarchs traverse over 3,000 miles to reach warmer climes in the Sierra Madre Mountains of Mexico. In the past two decades, North American monarch populations have plummeted. Once covering 20.97 hectares of overwintering habitat in 1996-1997, monarchs occupied a meager 0.67 ha in 2013-2014. This is a 97% decline in occupied land area. A staggering statistic, such losses are cause for concern. Some scientists and activists promote monarch butterfly protection under the United States Endangered Species Act. They argue that while species eradication is unlikely, we risk the loss of an incredible migratory event unparalleled on our continent. Before concrete conservation action can be implemented, it is crucial to ascertain the cause of these declines. According to a series of emails I received from Environment New York, “Monsanto is driving the monarch butterfly to the brink of extinction.” The subject merely reads: “Monsanto killing butterflies.” I found myself wondering “How?” but, more importantly, “Why?” The phrasing of these emails connoted a sense of malicious intent behind the actions of this controversial agricultural company. I imagined a group of nefarious individuals--executives, scientists, large-scale farmers—laughing maniacally as they ripped wings off butterflies amid a shower of money and herbicides pouring from the sky. This is hardly the reality of the situation. The real impetus behind Environment New York’s campaign against Monsanto rests in the reduction of common milkweed (a weed species commonly found in corn and soybean fields) as a direct result of herbicide use on genetically modified crops. Common milkweed is the preferred food source of monarch butterfly larvae. Migrating adults lay their eggs on plants interspersed throughout the Midwest every year. Farmers consider the plant a major pest species, however, as it reduces overall agricultural yields. They have always employed herbicides to decrease milkweed populations in their fields, but use of the most potent herbicide, glyphosate or RoundupTM (Monsanto), was previously limited due to its negative effects on crops. In the 1990s, Monsanto introduced genetically modified, glyphosate-tolerant corn and soybean plants to agricultural markets. By 2011, adoption of these crops reached 72% and 94%, respectively. Use of Roundup skyrocketed. Unsurprisingly, common milkweed populations crashed. Without common milkweed plants to feed their larvae, monarch butterflies were unable to adequately propagate their populations. The ever-increasing adoption of Monsanto’s Roundup ReadyTM crops during the past two decades matched increasing instances of monarch declines almost perfectly. It is important to note that the loss of milkweed is not the only stressor currently affecting monarchs. Extreme weather events (notably temperature fluctuations and changes in precipitation) associated with climate change and the loss of overwintering habitat at the hands of illegal loggers are also implicated in monarch declines. The stressors work synergistically. Evaluation of each factor’s relative impact on declines of butterfly populations, however, revealed that milkweed loss remains the primary cause. It appears Monsanto is killing butterflies. On March 31, 2015, Monsanto announced that it would contribute $3.6 million to the National Fish and Wildlife Foundation’s Monarch Butterfly Conservation Fund over the next three years. An additional $400,000 was included “to partner with and support the efforts of experts working to benefit monarch butterflies,” a companywide announcement read. An optimist may view this commitment as means to make amends – an attempt to right a wrong committed against butterflies. A cynic may see a carefully crafted public relations move that lacks long-term commitment to butterfly-friendly agricultural practice. Either way, Monsanto’s donation is a step in the right direction. Monarch butterflies can be saved, and individual citizens can absolutely aid these efforts. To raise awareness, reach out to government officials, herbicide companies, and farmers to let them know you care about the fate of butterflies. To actively aid conservation, plant local milkweed species on your property to provide nursery habitat for migrating monarch larvae. We are not powerless in the fight to save monarchs, and it is a battle worth fighting. They are an iconic American species: a source of inspiration for writers, artists, scientists, and children exploring their own natural world for the first time. Don’t let them down; don’t let monarchs fall. This blog post was originally published by Columbia Science Review in February 2015. By Alexandra L. DeCandia Humans have a love affair with plastic. Lightweight, versatile, durable, and inexpensive synthetic polymers have flooded the global market since 1950. Yet the qualities that earn success in the marketplace also severely endanger the natural environment. Winds, rivers, and currents ferry lightweight refuse ocean-bound, and cooler temperatures and UV-protection render it long lasting. Plastic floats in bodies of water for decades before degrading into “microplastics,” but even these miniscule particles pose risks to ocean life. They mingle with professional fishing gear and “ghost nets” that wander around the ocean silently seizing marine mammals. Because of these plastics, the limbs of aquatic life get entangled, digestive tracts occluded, and tissues infused with toxins. Entanglement occurs when marine mammals are constricted or entrapped by anthropogenic debris. This may lead to strangulation, open sores, impaired behaviors, increased energy expenditure, and, in extreme cases, drowning. For example, New Zealand fur seals get caught in stray lobster traps but aren’t strong enough to carry them to the surface; thus, they remain trapped. Similarly, Dugongs are unable to wriggle free from the constraints of fishing nets. Even humpback whales, if not freed from tows of nets, ropes, and plastics tangled around their flukes, sometimes die from exhaustion in their struggle to break free. Ocean debris acts as an anchor for these trapped animals. Even stray monofilament lines can lock animals down to the ocean floor, dooming them to death. The second threat stemming from the misuse of plastics, ingestion, occurs when organisms mistake debris for food. Plastics come in all shapes, sizes, and colors, so plastic debris can often mimic the look of mammalian food. Analysis of polar bear scat, for example, revealed ingestion of debris like foil, cardboard, cigarette butts, duct tape, foam rubber, glass, paint chips, paper, plastic, wood, and even a watch band. While these items luckily passed through the animal studied, others often do not. Since synthetic materials do not degrade as organic ones do, ingestion can often yield wounds both internally and externally, gastro-intestinal blockages, false sensations of satiation, toxin bioaccumulation in tissues, impaired feeding capability, and even starvation. In 2008, examination of the stomach contents of two sperm whales stranded in northern California uncovered ropes, plastics, and 134 different types of fishing nets. One whale died of a ruptured stomach; the other of starvation. Both deaths were directly caused by debris ingestion. Marine mammals are fairly diverse, so often times the distinct behaviors, morphologies, and habitat requirements render certain risks more threatening to one type of organism over another. The order Carnivora, which contains polar bears, sea otters, and pinnipeds, dwell at the intersection of land and sea. These animals seem to approach their dual environments with a sense of curiosity. As a result, marine debris poses a particular risk of entanglement as they excitedly explore their surroundings. Pinnipeds are drawn to novel items such as plastic bags or abandoned nets, and often accidentally slip their heads inside loops and holes. Then, since the animal is unable to escape, these “lethal necklaces” remain on the neck of the pinniped, constricting the animal as it grows. For some species, such as the critically endangered Hawaiian monk seal, entanglement has been implicated as a major threat to population growth. Even a seemingly modest rate of entanglement of 0.04%-0.78% has proven detrimental to an ailing population. The order Cetacea consists of mysticetes (baleen whales) and odontocetes (toothed whales). Unlike pinnipeds, cetaceans are entirely marine, large bodied, and migratory. Ingestion is therefore the greatest threat to these animals. Nets and plastics can become tangles at the bottom of the ocean and prevent them from feeding. On a smaller scale, microplastics join the krill that these animals ingest with every gulp or skim of the surface. When the animals filter feed, those synthetic particulates enter their bodies and, in the case of Mediterranean fin whales, leech toxins into their tissues. Odontocetes largely avoid these risks associated with filter feeding. However, their large-scale ingestion of marine debris does cause plastic impaction, gastro-intestinal tract blockages, starvation, and gastric rupture. The final order, Sirenia, contains dugongs and manatees. Like cetaceans, sirenians spend their entire lives submerged beneath the waves of aquatic environments. Unlike cetaceans, they travel through coastal waters, estuaries, and inland river networks. While herbivory minimizes the risk of microplastic bioaccumulation, entanglement and ingestion still pose a threat to these species. When we think about the popularity of plastics in our society, it seems that the threat of entanglement and ingestion is inevitable. However, through the implementation of stringent legislation, recycling initiatives, incentive programs, consumption reductions, and citizen-led clean up efforts, this does not have to be the case. All it takes is education, activism, and compliance on our part. While the ocean can never be completely devoid of plastic, we can prevent further degradation from this point on. Through increased awareness, it is possible that marine mammals won’t have to live in an environment inundated by trash. This blog post was originally published by Columbia Science Review in November 2014. By Alexandra L. DeCandia Humans have a talent for disrupting natural processes. Through the overharvest of species and inundation of landscapes with highways and suburbs, we’ve continuously rendered wild populations small and fragmented. Compared to larger, outbred populations, these communities exhibit higher rates of inbreeding. If their circumstances do not improve, inbreeding depression, or reduced reproductive fitness, may lock these populations in “extinction vortices,” whereby genetic and demographic declines work synergistically towards ultimate extinction. Luckily, it is possible to escape this vortex. “Genetic rescue,” a more positive example of human disruption, has been proposed as means of mitigating inbreeding depression. It occurs when immigrants into a small population drastically improve overall fitness beyond theoretical predictions. These immigrants inject genetic diversity into ailing populations and thereby reduce their genetic load by disrupting homozygous deleterious alleles. This enables population expansion and improved fitness, overall. As a management strategy, genetic rescue can be achieved in two ways: (1) facilitating natural gene flow through improved connectivity between fragmented populations, and (2) artificially translocating immigrants into an inbred population. The management of Mexican wolves presents an example of the first strategy. Mexican wolves (Canis lupus baileyi) are the most genetically distinct descendants of the North American gray wolf. Due to habitat loss and human hunting throughout the 19th and 20th centuries, these once abundant carnivores were reduced to seven captive individuals by the mid-20th century. Unsurprisingly, signs of inbreeding depression appeared in reintroduced populations as a result of founder effects and geographic isolation. To prevent further declines, conservation geneticists combined population viability analysis with topographic data to propose a series of corridors between the introduced populations. Increased connectivity, when combined with Mexican wolves’ dispersal capabilities, now facilitates natural gene flow between populations. Alternatively, the second management strategy, translocation, artificially transports foreign gametes or individuals into inbred populations. Source populations for these organisms include other wild populations (e.g. California bighorn sheep), captive populations (e.g. Houbara bustard), and wild populations of different subspecies (e.g. Florida panther). Genetically distinct, translocated individuals deposit variation from their source populations into those suffering from inbreeding depression. Occasionally, translocations even possess specific genotypic aims. In the case of the American chestnut (Castanea dentata), for example, conservation geneticists sought to imbue the once widespread species with genetic resistance to blight fungus. Through hybridization with the Chinese chestnut (C. mollissima) and repeated backcrossing with resistant American chestnuts, the species was able to maintain unique morphologies alongside appropriated fungal-resistance. While successful in the aforementioned cases, genetic rescue should not be considered a panacea for all species suffering from heavy genetic loads. As genetic management of wild populations remains relatively novel, few studies document its long-term effects. In some cases, the intentional hybridization of divergent populations can render hybrids and their offspring maladapted to a particular environment, a phenomenon termed outbreeding depression. These fitness reductions may even increase pathogen susceptibility, as was documented in an experimental crossing of two genetically distinct populations of largemouth bass (Micropterus salmoides). As a result, the decision to implement genetic rescue through connectivity or translocation becomes a cost-benefit analysis of the relative risks associated with inbreeding and outbreeding depression. What’s more, if the ultimate causes of a population’s decline are not removed, no number of translocations will be able to sustain the species in perpetuity. Despite these limitations, however, genetic rescue has proven a viable management strategy for highly inbred populations. Without it, the world would risk losing greater prairie chickens, Swedish adders, black-footed ferrets, freshwater mussels, South Island robins, golden lion tamarins, and a number of other species. Through improving connectivity and managing translocations of captive and wild individuals, humans are attempting to undo some of the damage we have inflicted upon the natural world. We are doing our part to aid in the escape from extinction vortices. This blog post was originally published in Columbia Science Review in April 2014. By: Alexandra L. DeCandia On February 17th, “little monsters” were in uproar: a poisonous primate had the audacity to bite Lady Gaga. A fuzzy prop in her latest music video, the offending slow loris nipped the star’s finger and was immediately returned to its box and carried away “in disgrace,” its role stricken from the video. The media loved this story. Due to the victim’s high profile and the aggressor’s status as the “cutest animal in the world,” the incident appeared in print, on television, and all over the Internet. With the exception of conservation blogs, the majority of coverage laughed it off as entertainment, joke-fodder, and a segue into listing tour dates. Within days, it was old news, and nothing more than yesterday’s anecdote. However, the story deserves more than cursory attention. The “disgraced” slow loris, reduced to nothing more than a badly behaved prop, is just one of thousands culled from the wild and forced into the exotic pet trade. A multi-billion dollar industry, this illegal trade of endangered species is fueled by YouTube videos (e.g. “tickling slow loris”) and uninformed celebrity endorsements (e.g. Gaga’s attempt to feature a loris in her music video) that are tickling species into extinction (Nekaris et al., 2013). All five species of slow loris (Javan, Bengal, Bornean, Sunda, and Pygmy) are considered Vulnerable or Endangered by the International Union for Conservation of Nature (IUCN). Nocturnal primates endemic to rainforests in South and Southeast Asia, they are primarily threatened by deforestation (i.e. habitat loss and fragmentation) and harvesting. While governments throughout their home range protect slow lorises from the latter, enforcement remains elusive. What’s more, despite their listing in Appendix I (which permits no trade) of the Convention on the International Trade of Endangered Species (CITES), slow lorises remain among the most commonly traded primates. They are sought for bush meat, traditional medicines, and exotic pets, and the demand is only strengthening. Unfortunately, slow loris biology and behavior render them particularly vulnerable to poaching. Their large eyes, crucial for hunting insects at night, brightly reflect flashlight beams. Their defense mechanisms, silent stillness and saliva-activated poison, afford little protection from harvesters. Their parenting style, “infant parking,” leaves poison-covered young hidden from predators but defenseless from humans. In these ways, slow lorises are sitting ducks, and in a market demanding their parts and tickles, they’re an attractive paycheck for poachers. There’s more cruelty to this story than mere capture, however. Medicine-bound slow lorises endure any number of horrors between forest and cabinet. Some creatures have their carcasses soaked in rice wine to create a tonic for easing pain during childbirth. Others are burned alive until their eyes burst, releasing minyak kukang, oil believed to restore vigor and incite love. Others still are dried on sticks and sold whole-bodied in the marketplace to be manipulated by the consumer. These practices are horrifying, yet they often occur right before the eyes of ambivalent law enforcement officials. In comparison to these medicinal practices, the pet trade may appear a more humane endeavor. However, the attrition rate of slow lorises in captivity reveals a different story. These animals are incredibly stress-sensitive. If they aren’t killed upfront by poachers separating mother from young, they often die in metal cages during transport from disease, malnutrition, or simply stress. In yet another display of cruelty, poachers even extract the slow loris’ teeth with pliers or nail clippers (and no anesthetic) to make them appear cuddlier to pet owners. This practice, pain notwithstanding, often causes young lorises to contract infections or even bleed to death. As a result of these practices, an estimated 30-90% of slow lorises captured for the pet trade die before ever reaching market. These numbers are astronomical and, unfortunately, not uncommon in the illegal trade of exotic wildlife. While possible, it is unlikely that the slow loris (almost) featured in Lady Gaga’s music video was bred in captivity. Like the thousands of other slow lorises in the pet trade, it was probably taken from the wild. Rather than the animal being “disgraced” by the incident, I argue that the humans involved should be blamed. The poacher who took the loris from its natural habitat, the middlemen who caged and smuggled it into the United States, the animal trainer who brought the animal to the shoot, and Lady Gaga, who sought to utilize the endangered creature as a prop, all took part in this multi-billion dollar industry built on the carcasses of exotic wildlife. Disgrace, in this instance, is theirs. Don’t join this queue of pain and suffering. If you see a video on YouTube featuring these creatures as pets, flag it. If you hear of a celebrity posing with an animal in Asia (as Rihanna did in 2009) or filming it in America (as Lady Gaga just did), condemn it. Finally, above all else, do not seek exotic wildlife as household pets. There are dozens of common, domesticated species that rely on humans for survival. Exotic animals are not among them. Leave slow lorises where they belong: in rainforests, not cages. This blog post was originally published in Columbia Science Review in February 2014. By Alexandra L. DeCandia The platypus (Ornithorhynchus anatinus) is a zoological enigma. One of three extant species in the basal order Monotremata, it exists as an amalgamation of inter-class characteristics that engender “Frankenstein’s monster” in taxonomic classification. Particularly in reproductive biology, platypodes blend the structures of mammals, reptiles, and birds to make for some of the strangest sex and reproduction in the animal kingdom. Unlike most other mammals, platypodes house their reproductive organs within a true cloaca. Retained from reptilian ancestors, this single aperture serves as both the exit point for excretory material and the exchange point for gametes. In females, the cloaca opens into a urogenital sinus that bifurcates into two reproductive tracts. On the left is a functioning ovary; on the right lies a small embryonic sac of primordial follicles. Such asymmetry, while common in many avian and some reptilian species, is rarely exhibited in mammals. It is matched only by the bifid penis of the male, which possesses asymmetrical glans that fit within the female’s uteri in a perfect “lock and key.” To aid fertilization, the upper third of each bifid is covered in keratinized spines that induce ovulation as well as flowery papillae that bring sperm closer to the follicles. While the bifid, spiny penis is the sexiest of platypus reproductive structures, it is not the only morphology that is a little different in monotreme males. They also possess ascrotal testes that reside within the abdomen. Termed “the testicondid condition,” platypodes are among birds, reptiles, and only a few other mammals that possess this primitive trait. Let it be known: despite their condition, male monotremes possess some of the largest testes in the world relative to their size. It is thought that sperm competition is likely the culprit. He whose testes are largest, produces the fastest sperm; he whose sperm swims fastest, fertilizes. Male platypodes do not rely solely on these massive testes to optimize their fitness. During breeding season, elevated testosterone levels induce more frequent male-male conflict as breeding partners are sought. Younger, more naïve males choose to adopt stealth as their strategy by exploiting temporal shifts in mate-seeking. Larger, more experienced males utilize battle as means of mate-acquisition. Ornithorhynchus may not appear a fierce warrior with his edentate bill and webbed feet, but with one swift jab of his hind leg, he can incapacitate any enemy long enough for mating to occur. Platypodes possess a venomous spur on the inside of each hind limb. One of the few extant venomous mammals, platypodes can use this nonlethal sting to establish a hierarchy between males and allow only the heartiest competitors to mate. Courtship in platypodes is brief and pointed. Males, having won the right to approach females unchallenged by subordinates, quickly mate and subsequently leave the inseminated female in search of other partners. The female, no longer interested in male attention, embarks on her nesting period. In yet another deviation from their mammalian counterparts, platypodes are oviparous. Once eggs reach roughly seventeen millimeters in utero, they exit the female’s cloaca and finish developing on the wet vegetation she deposited in her incubation chamber. Ten days later, the incredibly altricial young use their egg-teeth to liberate themselves from their parchment-shells in order to trade yolk-sacs for milk. Unlike all other mammals, monotremes lack nipples. Therefore, in a touching display of maternal devotion, the female sweats milk from her abdomen so her offspring may suckle and grow. Lactation lasts for roughly four months. In that time, young platypodes (charmingly called puggles) begin to resemble adults and reach a level of self-sufficient maturity befitting adolescents ready to take on the world. Once weaning has occurred with little ado, mother and young depart from the burrow. The males prepare to battle for dominance and inseminate as many females as possible with their keratinized, bifid glans. The females prepare to excavate riverbank burrows, lay eggs from their left uteri, and secrete milk for their young. It’s a unique, ancient strategy of reproduction that has stood the test of time among eutherian mammals alike, despite its strange and haphazard nature. It is successful, it is bizarre, and it is distinctly platypus. This blog post was originally published by Columbia Science Review in December 2013. By Alexandra L. DeCandia Popcorn and Caramel breathed easily this Thanksgiving. The two lucky turkeys chosen for the annual Presidential pardon observed the holiday at the White House both feathered and free from the indignities of stuffing. However, as the winner and runner-up of a turkey equivalent to the Hunger Games (as Obama quipped), Popcorn and Caramel are fortunate to share such an unusual fate. Every year, Americans consume an estimated 46 million turkeys on the Thanksgiving holiday – almost one-fifth of the annual U.S. production of 254 million birds. Such astronomical, gravy-soaked casualties reveal more than an American zeal for turkey. It’s become a celebratory obsession – a staple during those times of family, warmth, and togetherness. But how much do Americans really know about the coveted drumsticks and over-sized breasts they consume each year? A short trip through the wormholes of the Internet reveals more than just basting techniques and optimal cooking times. The modern North American turkey (Meleagris gallopavo) descended from a long line of feathered bipeds that originated in the Early Miocene (c. 23 million years ago). Belonging to the order of Galliformes, they are most closely related to grouse, quail, pheasants, partridges, and chickens, but maintain the largest body size. Equipped with elaborate plumage (capable of shining red, purple, green, and gold in males), a varied repertoire of gobbles, and a distinctly nubby red head (complete with the ever attractive wattle, snood, and caruncles), wild turkeys aren’t exactly the subtlest of creatures. Therefore, it was not long before man, that great tinker of life, sought its domestication. Archaeological evidence suggests that turkeys were first domesticated by the Mesoamerican indigenes roughly 2,000 years ago. Likewise a staple for native peoples in North America, wild and newly domesticated turkeys provided ample eggs, meat, and decorative feathers for centuries. However, with European colonization of the Americas came the export and ultimate industrialization of the turkey industry. More and more, these intense selective pressures led domesticated turkeys to display phenotypes altered from their wild cousins. They doubled in size, developed white feathers, and grew such large breast muscles that they lost the ability of flight (and even that of natural copulation). Perhaps their greatest divergence, though, was one of population size. While the domesticated turkey population exploded to meet the ever-growing demand of a burgeoning nation, that of wild turkeys greatly diminished due to gross overhunting and rampant logging that coupled America’s economic expansion. By the early 1900’s, wild turkey populations reached an all-time low of 30,000 individuals. Restricted to isolated pockets of their ancestral range and almost entirely extirpated from its extremes, the species seemed en route to extinction. However, through a combination of strict hunting regulations, frequent individual relocations, and newly acquired funds from the 1937 Pittman-Robertson Act (which reallocated money from firearm taxes to wildlife conservation), wild turkey populations were able to rebound at an exponential rate. Today, more than 7 million wild turkeys join the 254 million domesticated turkeys currently inhabiting the North American continent. Their numbers are strong, their range expanding, and their meat so delicious when paired with good company and the perfect stuffing. Even Ben Franklin realized the significance of such a distinctly American bird. As he penned in a letter to his daughter (leading to the apocryphal myth of his desire for a Galliform national bird): “The turkey is…a respectable bird, and withal a true original Native of America…He is besides, though a little vain and silly, a Bird of Courage.” So be mindful and proud of your turkey next Thanksgiving. Remember the awkward majesty of its appearance, the struggle of its existence, and the tradition of its place at the center of American life and celebration. Then, once all due respect has been paid, gobble it up with the greatest care and ample amounts of gravy. |
AboutWelcome to the EEBlog! This page links to blog posts DeCandia lab members have written about wildlife conservation & the environment. It also includes posts written for other outreach platforms. Please see linked websites for original versions, when applicable. Archives
February 2021
|